Chow Lab: Molecular Control of Regulated Secretion
| Email: | rchow@usc.edu |
| Office Location: | ZNI room 325 |
| Office Phone: | (323) 442-2901 |
| Lab Location: | ZNI 323 |
| Lab Phone: | (323) 442-0099 |
| Website: | Chow Lab |
| Ph.D. Programs: | PIBBS/Neuroscience |
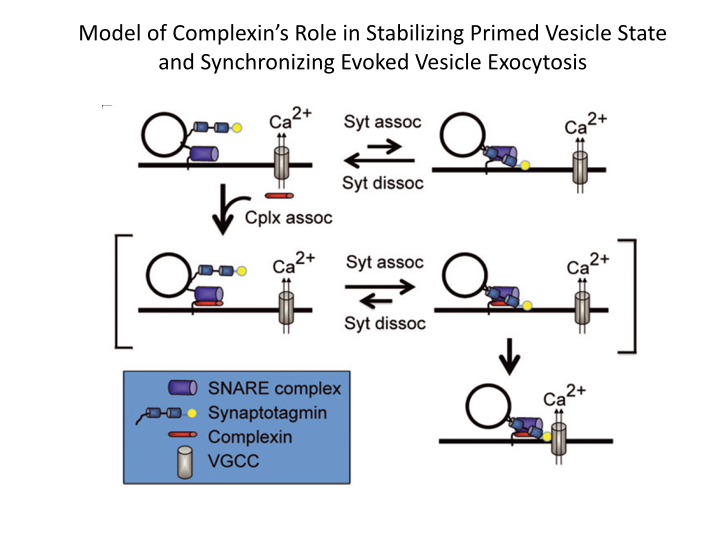
The aim of my laboratory is to advance our understanding of how hormone and neuronal secretion is controlled in normal and pathological states, and then to translate this knowledge to improve human health. In the last decade, neuroscience has benefited tremendously from the molecular biology revolution. Dozens of synaptic proteins have been cloned. One of the most exciting goals today is to figure out how these proteins orchestrate the complex life cycle of the secretory vesicle. It is of particular interest to understand how calcium is involved. Katz and colleagues showed that calcium triggers fast exocytosis. More recently it has become clear that calcium may play other important roles that precede exocytosis, perhaps facilitating translocation, docking and priming of vesicles.
We work with neurons and endocrine cells. Many endocrine cells such as pancreatic beta and chromaffin cells, like neurons, fire action potentials, exhibit fast calcium-dependent exocytosis, and express synaptic proteins or closely related proteins. They have the advantages of being easy to prepare, and their secretory vesicles are larger than synaptic vesicles and can therefore be visualized more readily. We use viral vectors to express the protein of interest in native or mutated form, and we then study the effects on secretion. The high-resolution methods we use to monitor exocytosis include membrane capacitance measurements, carbon-fiber amperometry, and total internal reflection fluorescence microscopy (also called evanescent-wave microscopy).
Another related area of interest of the laboratory is the cellular mechanism of abnormal secretion and cell death in diabetes mellitus. In collaboration with Prof. Ralf Langen (Biochemistry, ZNI), we are studying the mechanism of cell death in pancreatic beta cells. The aim is to understand the nature of derangements in beta cell function early in disease, which may permit design of therapeutic interventions early in the development of type I and II diabetes.
In collaboration with Mark Humayun (Ophthalmology, BME) and Jim Weiland (Ophthalmology, BME), we are studying methods to restore sight to the blind. In my laboratory, we perform imaging of retinal ganglion and bipolar neurons expressing genetically encoded calcium indicators, in order to better understand how to improve signaling between the microelectrode array of an implanted epiretinal prosthesis and the retina. In addition, we are working on photoactivated molecules that embed in cell membranes, converting the cells into light-sensors -- a novel cellular prosthesis.
A recent interest of our laboratory pertains to cancer biology. When cancer cells become invasive, they express many neuronal-type proteins, including those involved in electrical signaling, secretion, and motility. Thus, we have been studying how these genes are turned on, in an effort to develop approaches to recognize the potential of cancer cells to invade/metastasize, as well as to find ways to stop invasion. One recent discovery we made in collaboration with Prof. Kirk Shung (BME) is that highly invasive breast cancer cells, but not less invasive cells, exhibit calcium elevations upon ultrasound stimulation. We are investigating the pathways involved, and we are collaborating with oncologists to develop a possible clinical assay.