Haselwandter Lab: Theoretical Biological Physics

| Email: | cah77@usc.edu |
| Office Location: | SSC 213B, UPC |
| Office Phone: | 213-740-1115 |
| Lab Location: | SSC 213A, UPC |
| Lab Phone: | 213-821-5340 |
| Website: | www.haselwandterlab.com |
| Ph.D. Programs: | Physics & Astronomy, Biological Sciences |
Arriving at a mechanistic understanding of life in terms of physical laws represents one of the greatest scientific challenges we face today. Recent developments in biology and physics present us with a unique opportunity to meet this challenge. On the one hand, many fields of biology now produce increasingly quantitative experimental data. Such quantitative data demand a quantitative, predictive understanding which goes beyond the cartoon models traditionally employed in biology. On the other hand, over the past few decades great strides have been made in our physical understanding of the collective behavior exhibited by complex systems, equipping us with a language in which to formulate physical descriptions of living systems. It is to be expected that new fundamental laws of nature will be uncovered by seeking a physical understanding of the organizational principles underlying life, and that such a physical understanding of life will also yield novel approaches for the control of living systems.
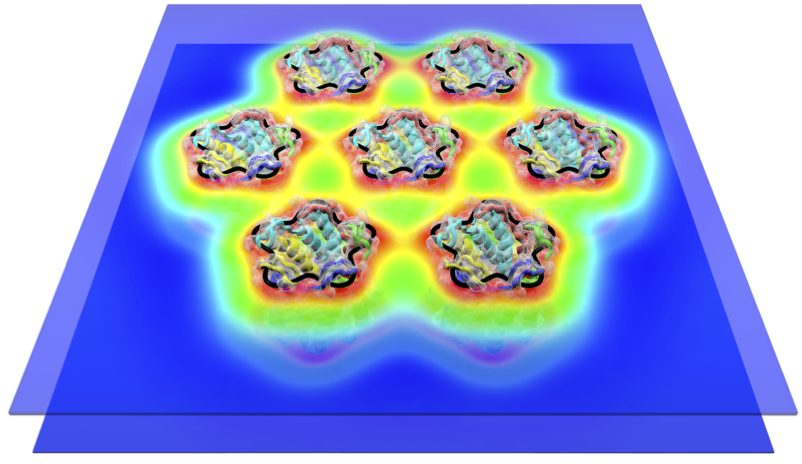
Fig. 1: Lipid bilayer deformations induced by a cluster of mechanosensitive ion channels.
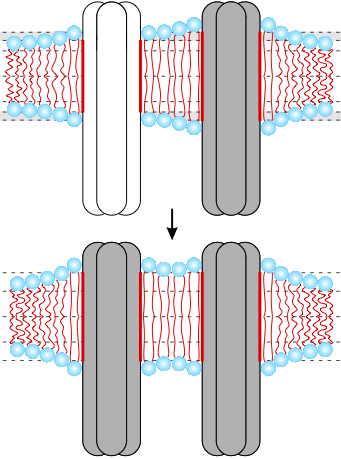
Fig. 2: Lipid bilayer-mediated cooperativity in chemoreceptor lattices.
In our research we endeavor to formulate simple physical descriptions of living systems involving very few (if any) free parameters. We employ such simple physical models to make quantitative predictions testing the relevance of a particular physical mechanism for a given biological phenomenon, with the overall aim of arriving at a physical understanding of complex biological data. The major thrust of our current research concerns the organization, dynamics, shape, and signaling properties of cell membranes. From a biological perspective, we mainly study the formation and regulation of membrane domains of synaptic receptor molecules, chemotactic signaling across cell membranes, and interactions between mechanosensitive membrane proteins and the surrounding lipid bilayer membrane.

